Gene therapy lacks tools to deliver in a safe and tissue-targeted manner. Limitations include toxicity, off-target effects due to targeting, and inability to repeat dose. We have developed a novel megakaryocyte-derived extracellular vesicle (MkEV)-based non-viral gene therapy delivery platform that preferentially targets bone marrow in vivo. Here we present work showing that STRM.BIO MkEVs: 1) pass through the liver and spleen to preferentially target bone marrow in mice and non-human primates (NHPs); 2) can selectively deliver pDNA cargo to long term HSCs to drive reporter protein expression exclusively in bone marrow following intravenous delivery in mice; and 3) present a platform to develop and deliver targeted gene therapies in vivo that are safe for repeat dosing. Our vision is to open the door to the future of medicine for patients living with rare diseases worldwide and bring gene therapy to life.
Primary human CD34+ hematopoietic stem and progenitor cells (hHSPCs) were differentiated into megakaryocytes in vitro. MkEVs were harvested and loaded with pDNA via electroporation, followed by DNase treatment to remove free cargo. For in vivo biodistribution and cargo-mediated protein expression studies, STRM.BIO MkEVs were exogenously labeled with fluorescent dye (PKH26, Cell Tracker Deep Red, and/or DiD), loaded with cargo, and IV-injected into mouse tail veins (wild type or NSG) or NHPs (cynomolgus monkeys). Tissues were collected 16-48h after IV injection, with MkEV tissue biodistribution quantified by dye fluorescence (Mean Fluorescence Intensity (MFI), plate reader) normalized to tissue weight or genomic DNA input, and pDNA cargo quantified by qPCR. Additionally, quantification of MkEVs+ cells was performed by flow cytometry analysis of murine bone marrow derived sub-populations (lineage negative, c-Kit +, Sca-1 + (LSK); lineage positive (Lin +); and, long-term HSCs (LT-HSCs; Lin -/CD150 +/CD201 +)) cells. Protein expression from MkEV-loaded pDNA cargo was similarly determined by plate reader fluorescence data and confocal microscopy.
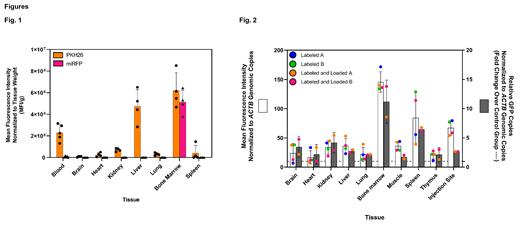
In mouse biodistribution studies, DiD-labeled MkEVs were predominantly detected in bone marrow, followed by liver and spleen 16h-post tail vein injection (plate reader analyses: Bone Marrow - 1.1e 4 ± 0.2 SD; Liver - 9.6e 3 ± 1.0 SD; Spleen - 1.8e 4 ± 0.2 SD. MFI/g tissue; n=3mice/group). Flow cytometry analyses show MkEVs preferentially targeted the hematopoietic compartment (of the MkEV+ cells, 94 ± 1% CD45+ vs 6 ± 1% CD45-, respectively), specifically, the HSPCs (68%, LSK) and LT-HSCs (100%) 48h post intravenous delivery via tail vein injection. Pharmacokinetic studies show that while MkEVs can circulate through highly vascularized tissues like the liver and spleen, they accumulate in the bone marrow. Strikingly, miRFP encoded by the pDNA was exclusively detected in bone marrow (p<0.0001 vs. all other tissues; Fig. 1).
In NHPs, dye-labeled MkEVSs were loaded with a MkEV-driven eGFP reporter plasmid. Studies showed preferential biodistribution to bone marrow, and preferential qPCR amplification of the pDNA cargo in bone marrow (both MFI/ ACTB and GFP/ ACTB were significantly higher vs. all other tissues: range p<0.05 through p<0.0001), Fig. 2. A tolerability and repeat dosing study was performed in NHPs. Monkeys were injected with 4 weekly doses of STRM.BIO MkEVs and serial bloodwork was obtained 6h post dose for cytokine evaluation and 5 days post dose for end organ damage assessment (n=4). The study showed no evidence of kidney, hematologic, or liver damage as evidenced by normal creatinine and electrolytes, no significant changes in complete blood counts, coagulation parameters, or liver function tests. There was no evidence of significant cytokine release or inflammatory changes on tissue histology. These data confirm that MkEVs are non-toxic and the platform is amenable to repeat dosing.
Taken together, these data establish an advanced gene therapy platform that can target bone marrow specifically following in vivo administration in both mice and NHPs, potentially eliminating the need for current ex vivo approaches to treat rare blood disorders, and providing the critical ability to repeat dose as required in clinic. STRM.BIO is leveraging our platform to develop gene therapies for rare blood diseases in people.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal